1.Chemical Equations
A chemical equation is a statement or representation that describes a chemical reaction in terms of
symbol and formulae.
➢ A chemical reaction is the transformation of chemical substance called reactants into another chemical
substance called products. In a chemical reaction, only rearrangement of atoms takes place.
➢ The substances which take part in a chemical reaction are called reactants. The reactants are written
on the left hand side. The new substances produced as a result of chemical reaction are called
products. The products are written on the right hand side.
Some of the symbols used in a chemical Equation are :
(a) Solids (s)
(b) Liquids (l)
(c) Gases (g)
(d) Aqueous solutions (aq)
(e) Gas released as a product (↑)
(f) Precipitate formed in the reaction (↓)
(g) Direction of reaction (→)
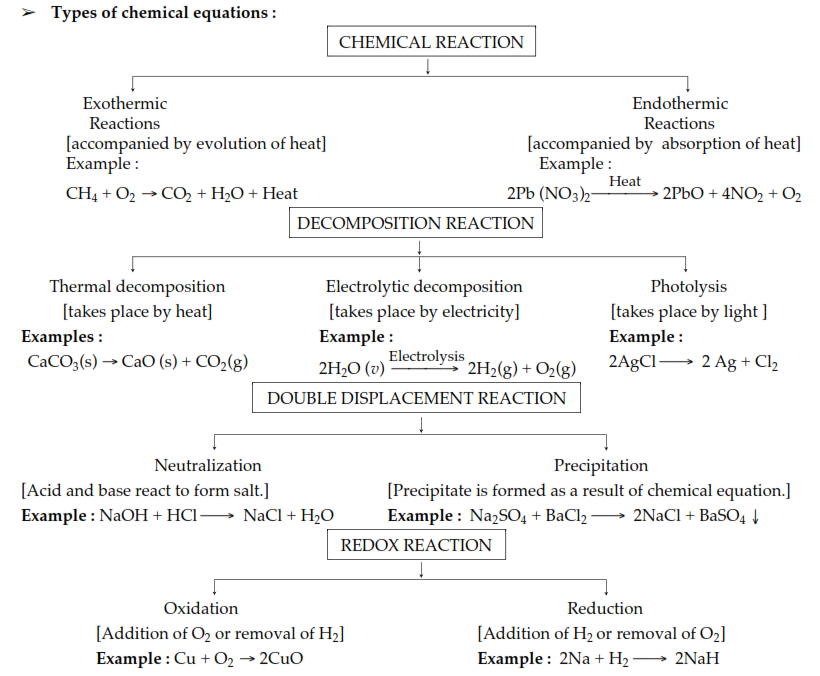
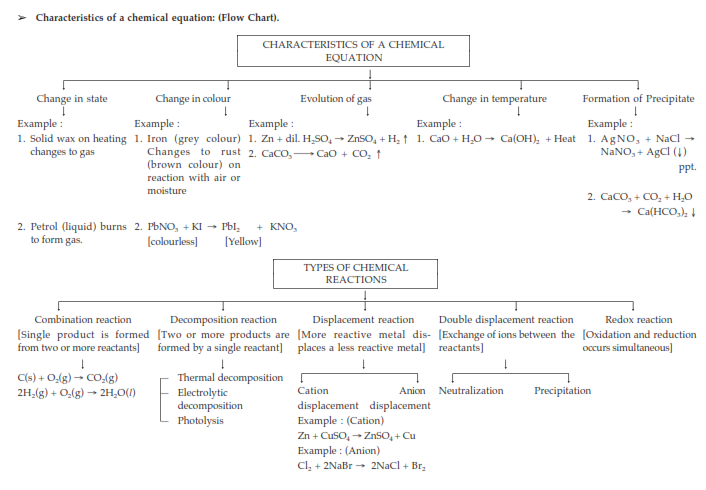
➢ Steps to balance a chemical equation :
(a) Write word equation.
(b) Then write skeletal chemical equation.
(c) Enclose the formula in the boxes.
(d) List the number of atoms of different elements present in the unbalanced equation.
(e) Start balancing with the compound that contains the maximum number of atoms.
(f) Start balancing other atoms.
(g) Check the correctness of the balanced equation.

 Carrier Point
Carrier Point
