- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Occurrence of Metals
Metals are generally found in the earth’s crust.
Elements or compounds which occur naturally in earth crust are known as Minerals. Minerals from which pure metals can be extracted are known as Mineral Ores.
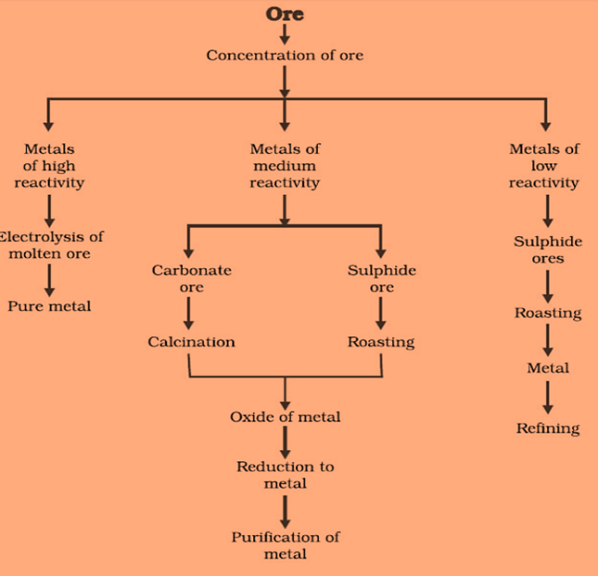
Steps for extraction of metals from its ore are:
- The first step is the enrichment of the ore
- The second step includes extraction of metals
- Third steps involve refining of metal
Gangue - Ores contain different impurities in it such as sand, soil etc. These impurities are known as Gangue.
Extracting Metals which are low in activity series
Metals which are low in the activity series are unreactive. The oxides of such metals can be reduced to metals by heating alone. For Example, Cinnabar (HgS)

Extracting Metals in the middle of the Activity Series
These metals are moderately reactive. They exist as sulphides or carbonates in nature. Before reduction, metal sulphides and carbonates must be converted into metal oxides.by heating strongly in the presence of excess air, this is known as Roasting. Carbonate ores are converted into oxides by heating in limited air. This is known as Calcination.
Roasting

Calcination

Reduction-metal oxides can be reduced to metals using a reducing agent such as Carbon.
Extracting metals towards the top of the activity series
The metals are highly reactive. They cannot be obtained by heating. For Example, Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
At cathode Na+ + e- → Na
At anode 2Cl- → Cl2 + 2e-
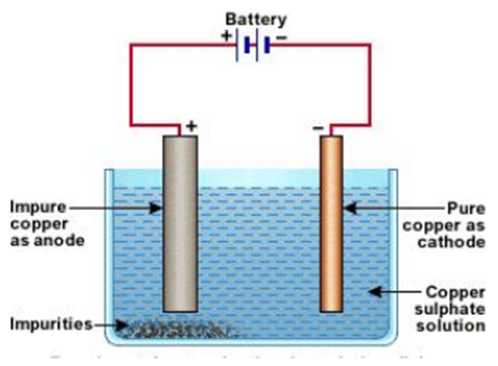
Refining of metals
Refining of impure metal is done using electrolytic refining. Impure copper is used as anode and a strip of pure copper is used as Cathode. Acidified copper sulphate is used as an electrolyte. When an electric current is passed through this, impure metal from the anode gets deposited in the electrolyte solution, whereas pure metal from the electrolyte is deposited at the cathode.
Deposition of insoluble residue formed from the dissolution of the anode during commercial electrolysis.

 Vaishnav Publication
Vaishnav Publication
