- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Ethanol and Ethanoic Acid
Ethanol
(i) Ethanol, C2H5OH is a colourless liquid having a pleasant smell.
(ii) It boils at 351 K.
(iii) It is miscible with water in all proportions.
Uses:
1. As a solvent in the manufacture of paints, dyes, medicines, soaps and synthetic rubber.
2. As a solvent to prepare the tincture of iodine.
How Do Alcohols Affect Human Beings?
(i) It causes addiction, damages the liver if taken in excess.
(ii) High consumption of ethanol may even cause death.
Reactions of Ethanol with Sodium
Ethanol reacts with sodium to produce hydrogen gas and sodium ethoxide. This reaction supports the acidic character of ethanol.
2C2H5OH+2Na → 2C2H5ONa+H2(↑)
Elimination Reaction
An elimination reaction is a type of reaction in which two substituents are removed from a molecule. These reactions play an important role in the preparation of alkenes.
Dehydration Reaction
Ethanol reacts with concentrated sulphuric acid at 443 K to produce ethylene. This reaction is known as dehydration of ethanol because, in this reaction, a water molecule is removed from the ethanol molecule.
CH3CH2OH → CH2=CH2+H2O
(reaction taking place in presence of Conc.H2SO4)
Ethanoic Acid or Acetic Acid
(i) Molecular formula: CH3COOH
(ii) It dissolves in water, alcohol and ether.
Esterification
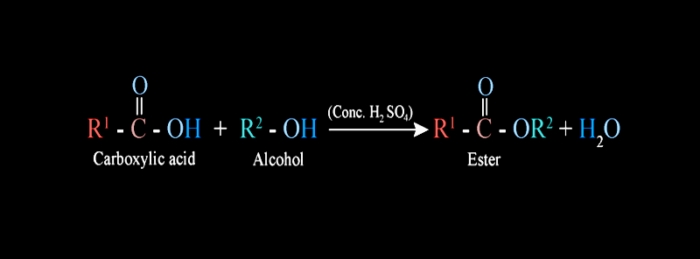
SaponificationWhen a carboxylic acid is refluxed with alcohol in the presence of a small quantity of conc.H2SO4, a sweet-smelling ester is formed. This reaction of ester formation is called esterification.
A soap is a sodium or potassium salt of long-chain carboxylic acids (fatty acid). The soap molecule is generally represented as RCOONa, where R = non-ionic hydrocarbon group and −COO−Na+ ionic group. 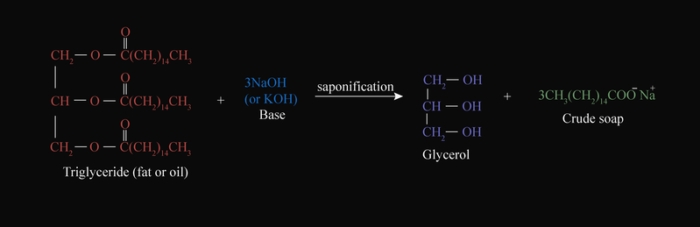
Reaction of Ethanoic Acid with Metals and Bases
Ethanoic acid (Acetic acid) reacts with metals like sodium, zinc and magnesium to liberate hydrogen gas.
2CH3COOH+2Na→2CH3COONa+H2(↑)
It reacts with a solution of sodium hydroxide to form sodium ethanoate and water.
CH3COOH+NaOH→CH3COONa+H2O
Carboxylic acids react with carbonates and bicarbonates with the evolution of CO2 gas. For example, when ethanoic acid (acetic acid) reacts with sodium carbonate and sodium bicarbonate, CO2 gas is evolved.
2CH3COOH+Na2CO3→2CH3COONa+H2O+CO2
CH3COOH+NaHCO3→CH3COONa+H2O+CO2
Friendly Carbon
Why Carbon Can Form so Many Compounds
Catenation occurs most readily with carbon due to its small size, electronic configuration and unique strength of carbon-carbon bonds. Tetravalency, catenation and tendency to form multiple bonds with other atoms account for the formation of innumerable carbon compounds.
Catenation
Catenation is the self-linking property of an element by which an atom forms covalent bonds with the other atoms of the same element to form straight or branched chains and rings of different sizes. It is shown by carbon, sulphur and silicon.
S8
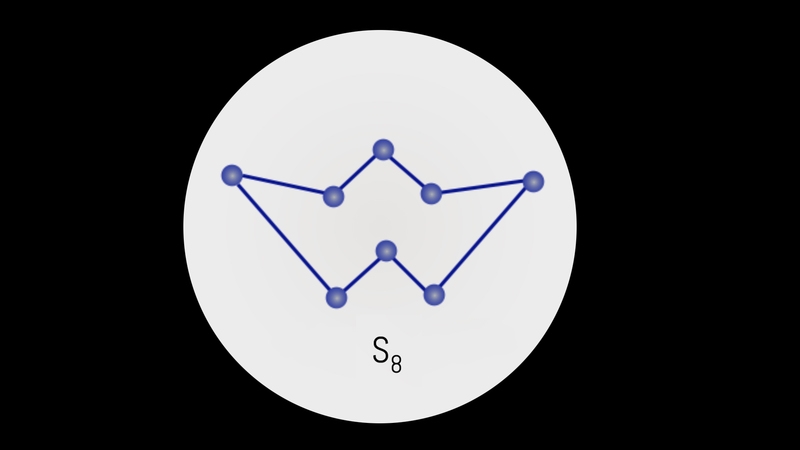
In its native state, sulphur show catenation up to 8 atoms in the form of S8 molecule. It

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
