- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
METALS AND NON- METALS REACTIONS
- Atoms of the metals lose electron from their valence shell to form cation.
- Atoms of the non- metals gain electrons in the valence to form anion.
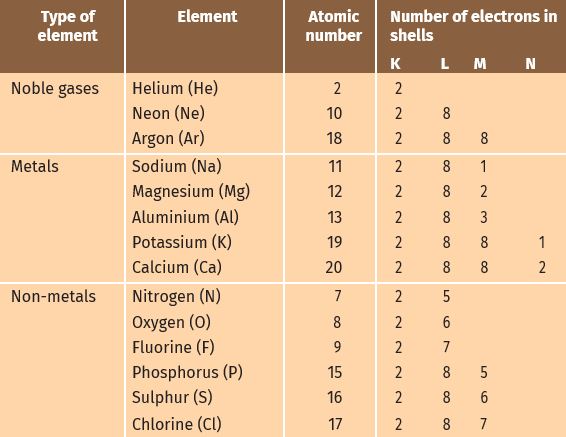
Formation of sodium chloride.
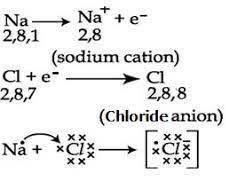
Formation of magnesium chloride
Properties of ionic compound
- PHYSICAL NATURE :- They are solid and hard (because of the strong force of attraction between the positive and negative ions) . They are brittle.
- Melting and boiling point:_ They have high melting and boiling pont.
- Solubility :- soluble in water and insoluble in solvents such as kerosene , petrol etc.
- Conductor of electricity :- Ionic compound conduct electricity in molter (ions move to the opposite electrodes when electricity is passed )
- They do not conduct electricity in solid state as movements of ions is not possible in solid They conduct electricity in molten state.

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
