- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Physical Properties of Organic Compounds
Most of the organic compounds have low boiling and melting point, due to the weak force of attraction (i.e., the inter-molecular force of attraction) between these molecules. Most carbon compounds are poor conductors of electricity, due to the absence of free electrons and free ions.
Chains, Branches and Rings
Saturated and Unsaturated Hydrocarbons
Saturated hydrocarbons: These hydrocarbons have all carbon-carbon single bonds. These are known as alkanes. General formula = CnH2n+2 where n = 1, 2, 3, 4.…..
Unsaturated hydrocarbons: These hydrocarbons have at least one carbon-carbon double or triple bond.
Hydrocarbons with at least one carbon-carbon double bond are called alkenes. General formula = CnH2n where n = 2, 3, 4…..
Hydrocarbons with at least one carbon-carbon triple bond are called alkynes. General formula = CnH2n−2 where n = 2, 3, 4…..
Chains, Rings and Branches
Carbon chains may be in the form of straight chains, branched chains or rings.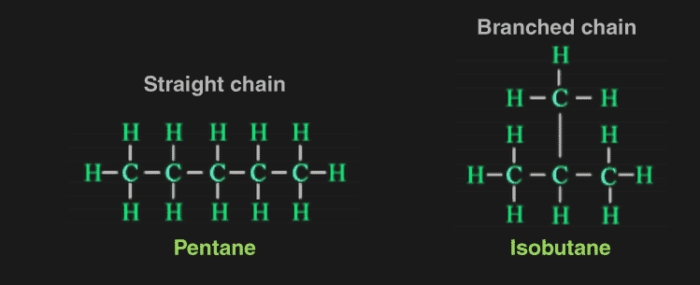
In cyclic compounds,
Structural Isomers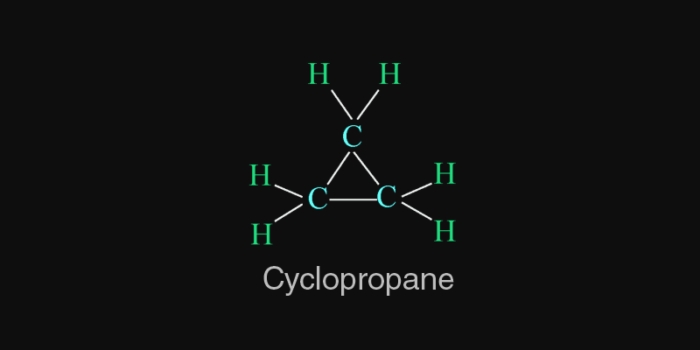
The compounds with the same molecular formula and different physical or chemical properties are known as isomers and the phenomenon is known as isomerism.
The isomers that differ in the structural arrangement of atoms in their molecules are called structural isomers and the phenomenon is known as structural isomerism.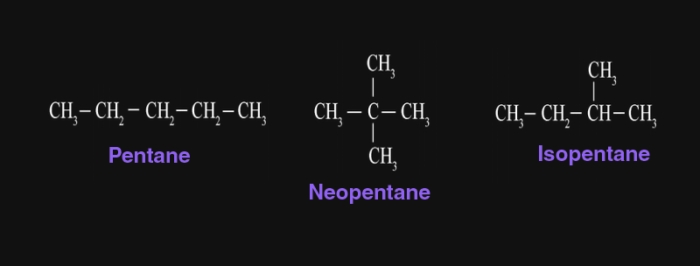
Benzene
Benzene is the simplest organic, aromatic hydrocarbon.
Physical properties: colourless liquid, pungent odour, flammable, volatile.
Structure:
Cyclic in nature with chemical formula, C6H6, i.e., each carbon atom in benzene is arranged in a six-membered ring and is bonded to only one hydrogen atom.
It includes 3-double bonds which are separated by a single bond.
Hence, this arrangement is recognized to have conjugated double bonds and two stable resonance structures exist for the ring.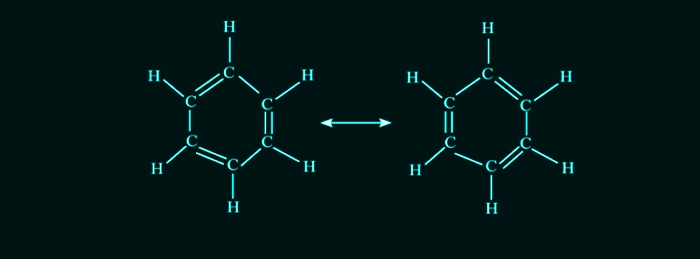
Functional Groups and Nomenclature
Functional Groups
An atom or a group of atoms which when present in a compound gives specific physical and chemical properties to it regardless of the length and nature of the carbon chain is called a functional group.
Classification of Functional Groups
Main Functional Groups:
(i) Hydroxyl group (-OH): All organic compounds containing -OH group are known as alcohols. For example, Methanol (CH3OH), Ethanol (CH3−CH2−OH), etc.
(ii) Aldehyde group (-CHO): All organic compounds containing -CHO group are known as aldehydes. For example, Methanal (HCHO), Ethanal (CH3CHO), etc.
(iii) Ketone group (-C=O): All organic compounds containing (-C=O) group flanked by two alkyl groups are known as ketones. For example, Propanone (CH3COCH3), Butanone (CH3COCH2CH3), etc.
(iv) Carboxyl group (-COOH): All organic acids contain a carboxyl group (-COOH). Hence, they are also called carboxylic acids.
For example, Ethanoic acid (CH3COOH), Propanoic acid (CH3CH2COOH), etc.
(v) Halogen group (F, CI, Br, I): The alkanes in which one or more than one hydrogen atom is substituted by- X (F, CI, Br or I) are known as haloalkanes. For example, Chloromethane (CH3Cl), Bromomethane (CH3Br), etc.
Homologous Series
Homologous series constitutes organic compounds with the same general formula, similar chemical characteristics but different physical properties. The adjacent members differ in their molecular formula by −CH2.
Physical Properties
The members of any particular family have almost identical chemical properties due to the same functional group. Their physical properties such as melting point, boiling point, density, etc., show a regular gradation with the increase in the molecular mass.

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
