- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
Mendeleev’s Periodic Table
Mendeleev arranged 63 elements known at that time in the periodic table. According to Mendeleev “ the properties of the elements are a periodic function of their atomic masses” the table consists of eight vertical coloumn called groups and horizontal rows called periods.
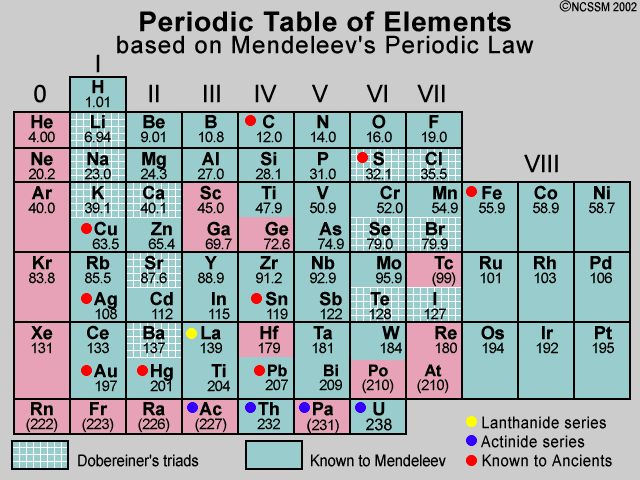
Achievements :-
(I) The arrangement of elements in group and periods made the study of elements quite systematic in the sence that if properties of one element in a particular group are known, those of the other can be easily .
(II) many gaps were left in this table for undiscovered elements however, properties of these elements could be predicted in advance from their expected position. This helped in the discovery of these elements the lements silicon , gallium and germanium were ddiscovered in this manner.
(III) Mendeleev corrected the atomic masses of certain elements with the help of their excepted position and properties.
(Iv) When inert gasses were discovered they could be placed in a new group without disturbing the existing order.
Limitations :-
- He could not assign a correct position of hydrogen in his periodic table 1 as the properties of hydrogen resembles both with alkali metals as well as with halogens.
- The atomic masses do not increases a regular manner in going from one elements to the next , so it was not possible to predict how many elements could be discovered between two elements.
- The isotopes of some element will be given different position if atomic number is taken as basis which will disturb the symmetry of the periodic table.

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
