- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Mendeleev’s Periodic Table and Law
The physical and chemical properties of elements are periodic functions of their atomic weights.
Features of Mendeleev’s Periodic Table
● Twelve horizontal rows, which were condensed to 7, known as periods.
● Eight vertical columns known as groups.
● Groups I to VII subdivided into A and B subgroups.
● Group VIII doesn’t have any subgroups and contains three elements in each row.
● Elements in the same group exhibit similar properties.
Achievements of Mendeleev’s Periodic Table
1. A systematic study of elements: Elements with similar properties were grouped together, that made the study of their chemical and physical properties easier.
2. Correction of atomic masses: Placement of elements in Mendeleev’s periodic table helped in correcting the atomic masses of certain elements. For example, the atomic mass of beryllium was corrected from 13.5 to 9. Similarly, atomic masses of indium, gold, platinum etc., were also corrected.
Limitations of Mendeleev’s Periodic Table
1. Position of hydrogen: Hydrogen resembles both, the alkali metals (IA) and the halogens (VIIA) in properties, so, Mendeleev could not justify its position.
2. Position of isotopes: Atomic weight of isotopes differ, but, they were not placed in different positions in Mendeleev’s periodic table.
3. Anomalous pairs of elements: Cobalt (Co) has higher atomic weights but was placed before Nickel (Ni) in the periodic table.
4. Placement of like elements in different groups: Platinum (Pt) and Gold (Au) have similar properties but were placed in different groups.
5. Cause of periodicity: He could not explain the cause of periodicity among the elements.
Periodicity of Properties: The repetition of properties of elements after certain regular intervals is known as Periodicity of Properties.
Merits of Mendeleev’s Periodic Table
1.Mendeleev’s left vacant places in his table which provided an idea for the discovery of new elements. Example: Eka-boron, Eka-aluminium and Eka-silicon.
2.Mendeleev’s periodic table was predicted properties of several undiscovered elements on the basis of their position in Mendeleev’s periodic table.
3.It is useful in correcting the doubtful atomic masses of some elements.
4.Noble gases could accommodate in the Mendeleev’s periodic table without disturbing the periodic table after discovery.
Limitations of Mendeleev’s Periodic Table
(a) No fixed position for hydrogen: No correct position of the hydrogen atom was in Mendeleev’s periodic table.
Example: Position of hydrogen with alkali metals and halogens (17th group).
(b) No place for isotopes: Position of isotopes were not decided.
Example: Cl-35 and Cl-37.
(c) No regular trend in atomic mass: Position of some elements with lower atomic masses before with higher atomic mass.
Example: Ni-58.7 before Co-58.9.
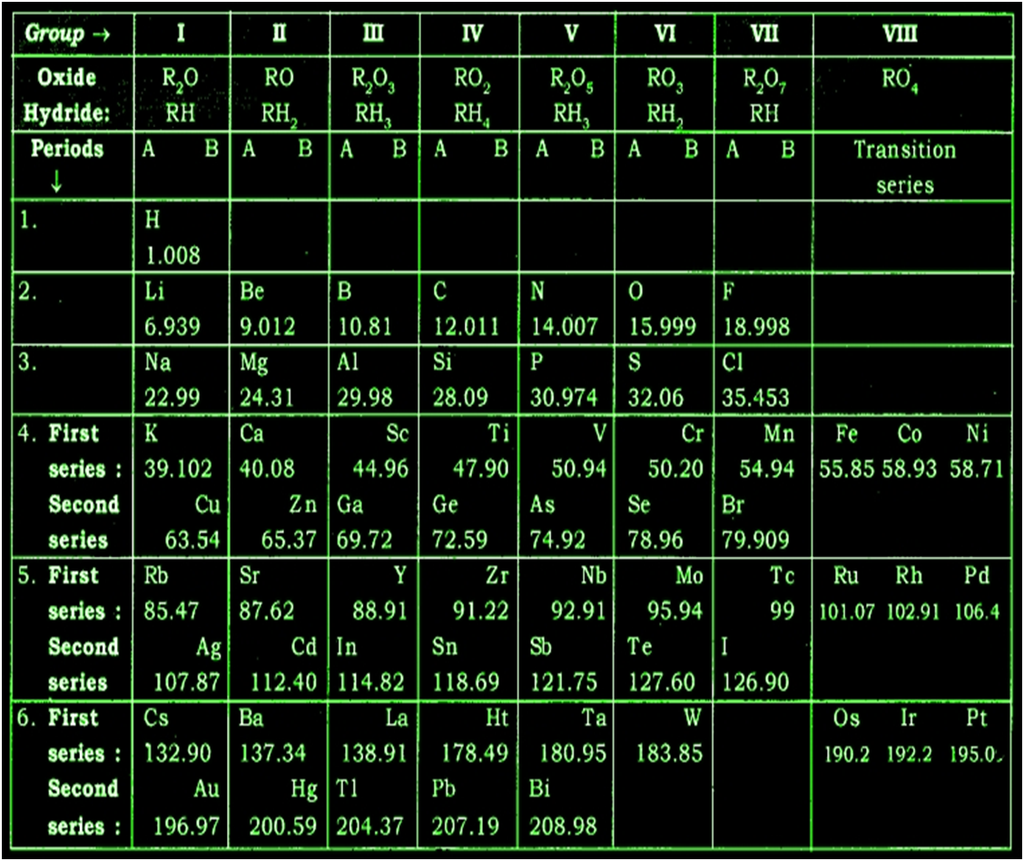
Mendeleev’s original periodic table is reproduced in the table below

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
