- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
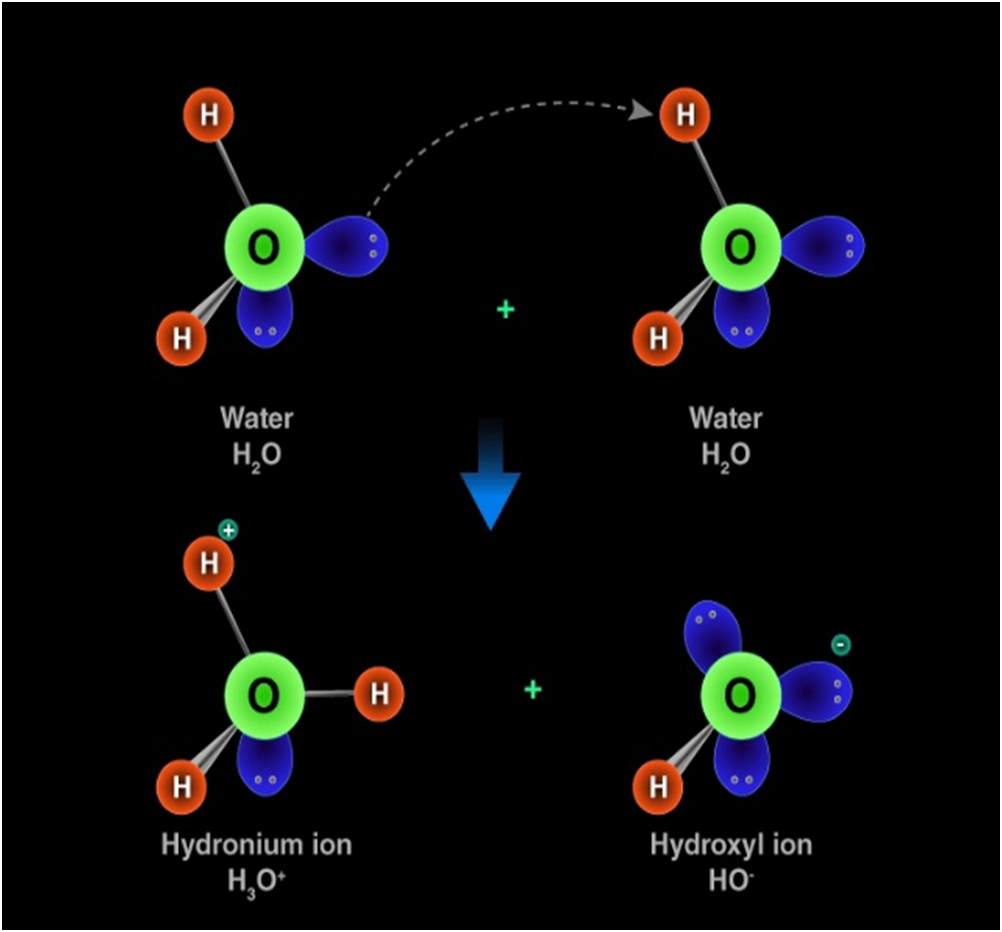
Hydronium ion
Hydronium ion is formed when a hydrogen ion accepts a lone pair of electrons from the oxygen atom of a water molecule, forming a coordinate covalent bond.
Dilution
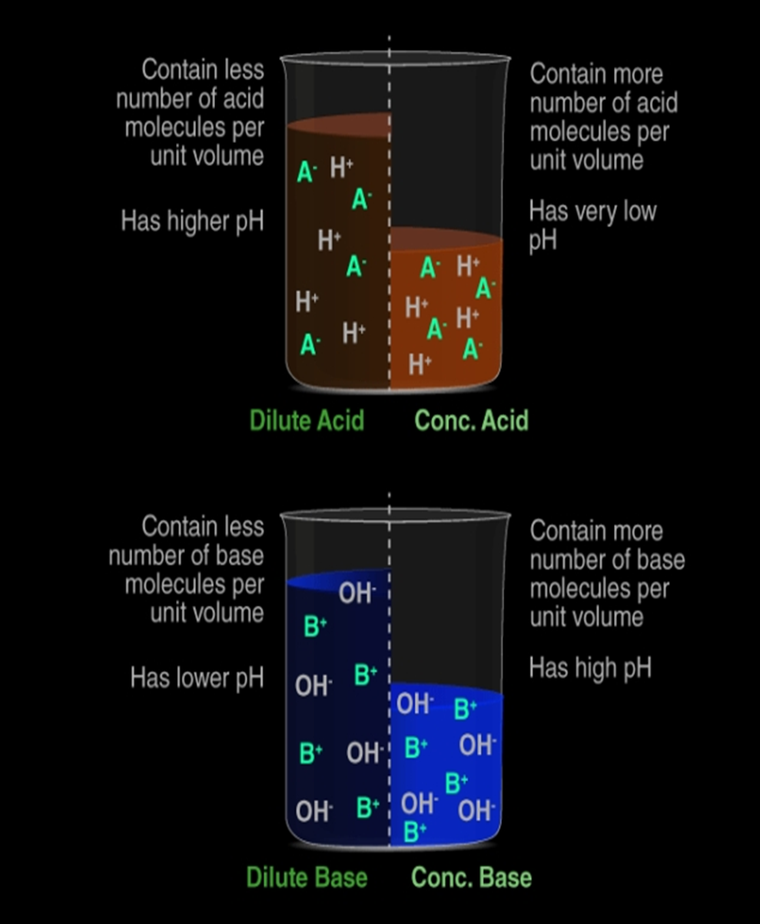
Dilution is the process of reducing the concentration of a solution by adding more solvent (usually water) to it.
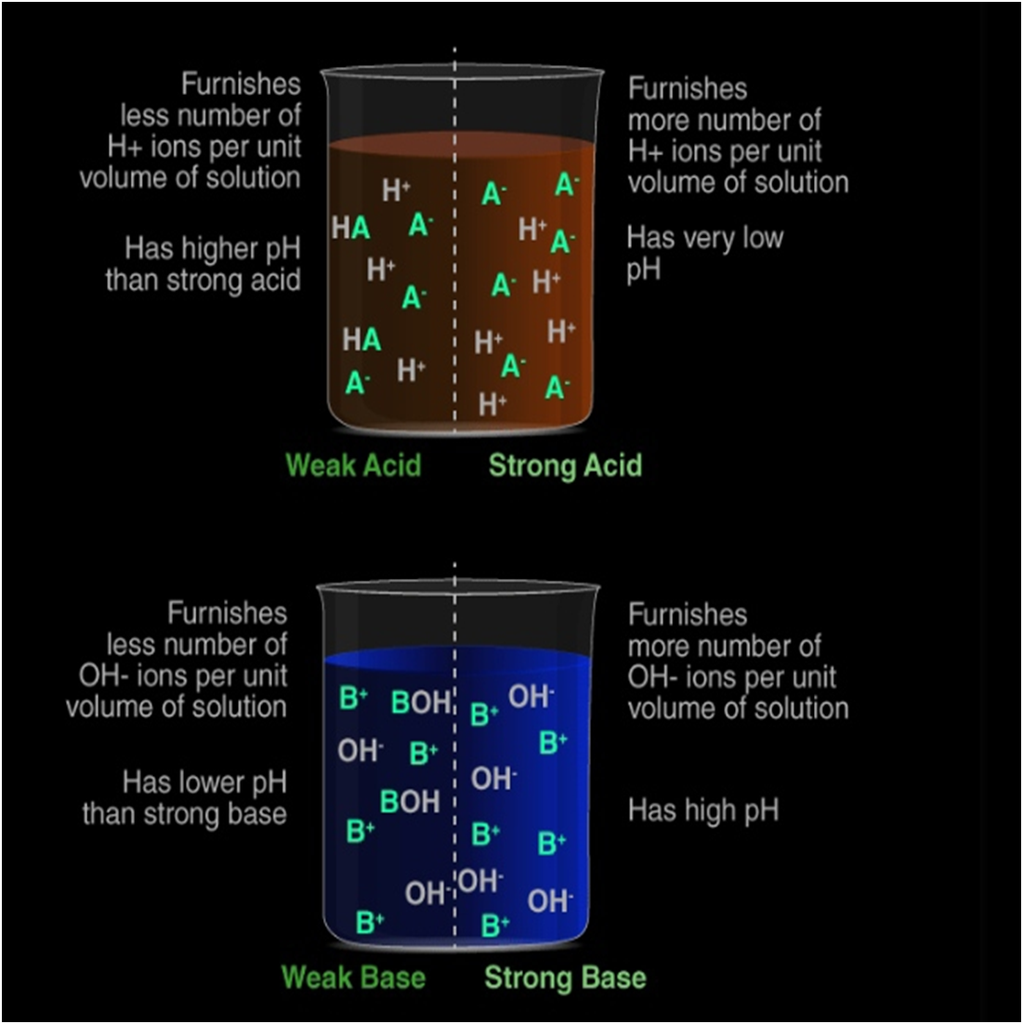
It is a highly exothermic process. Strong acid or base: When all molecules of a given amount of an acid or a base dissociate completely in water to furnish their respective ions, H+(aq) for acid and OH−(aq) for base).
Weak acid or base: When only a few of the molecules of a given amount of an acid or a base dissociate in water to furnish their respective ions, H+(aq) for acid and OH−(aq) for base).
Dilute acid: contains less number of H+(aq) ions per unit volume.
Concentrated acid: contains more number of H+(aq) ions per unit volume

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
