- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Indicators: Indicators are substances which indicate the acidic or basic nature of the solution by the colour change.
Types of Indicator: Some common types of indicators are:
1. Natural Indicators: Indicators obtained from natural sources are called Natural Indicators. Litmus, turmeric, red cabbage, China rose, etc., are some common natural indicators used widely to show the acidic or basic character of substances.
Turmeric: Turmeric is another natural indicator. Turmeric is yellow in colour. Turmeric solution or paper turns reddish brown with base. Turmeric does not change colour with acid.
Red Cabbage: The juice of red cabbage is originally purple in colour.
2. Olfactory Indicator: Substances which change their smell when mixed with acid or base are known as Olfactory Indicators. For example; Onion, vanilla etc.
Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid.
Vanilla: The smell of vanilla vanishes with base, but its smell does not vanish with an acid.
3. Synthetic Indicator: Indicators that are synthesized in the laboratory are known as Synthetic Indicators. For example; Phenolphthalein, methyl orange, etc.
Phenolphthalein is a colour less liquid. It remains colour less with acid but turns into pink with a base.
Methyl orange is originally orange in colour. It turns into the red with acid and turns into yellow with base.
Acids, Bases and Salts
Classification of matter
On the basis of
a) composition – elements, compounds and mixtures
b) state – solids, liquids and gases
c) solubility – suspensions, colloids and solutions
Types of mixtures – homogeneous and heterogeneous
Types of compounds – covalent and ionic
an Acid and a Base?
Ionisable and non-ionisable compounds
An ionisable compound when dissolved in water or in its molten state, dissociates into ions almost entirely. Example: NaCl, HCl, KOH, etc.
A non-ionisable compound does not dissociate into ions when dissolved in water or in its molten state. Example: glucose, acetone, etc.
Arrhenius theory of acids and bases
Acids: Acids are sour in taste, turn blue litmus red, and dissolve in water to release H+ ions.
Properties of Acids:
Acids have a sour taste.
Turns blue litmus red.
Acid solution conducts electricity.
Release H+ ions in aqueous solution.
Types of Acids: Acids are divided into two types on the basis of their occurrence i.e., Natural acids and Mineral acids.
(i) Natural Acids: Acids which are obtained from natural sources are called Natural Acids or Organic Acids.
Examples:
Methanoic acid (HCOOH)
Acetic acid (CH3COOH)
Oxalic acid (C2H2O4) etc.
(ii) Mineral Acids: Acids that are prepared from minerals are known as Mineral Acids Example; Inorganic acids, man-made acids or synthetic acid are also known as Mineral Acids.
Example:
Nitric acid (HNO3)
Carbonic acid (H2CO3)
Phosphoric acid (H3PO4) etc.
Chemical Properties of Acid:
(i) Reaction of acids with metal: Acids give hydrogen gas along with respective salt when they react with a metal.

Metal + Acid → Salt + Hydrogen
Test For Hydrogen Gas: The gas evolved after reaction of acid with metal can be tested by bringing a lighted candle near it. If the gas bums with a pop sound, then it confirms the evolution of hydrogen gas.
(ii) Reaction of acids with metal carbonate: Acids give carbon dioxide gas and respective salts along with water when they react with metal carbonates.
![]()
Metal carbonate + Acid → Salt + Carbon dioxide + Water

(iii) Reaction of acid with hydrogen carbonates (bicarbonates): Acids give carbon dioxide gas, respective salt and water when they react with metal hydrogen carbonate.

Example:
Test For Evolution of Carbon Dioxide Gas: Carbon dioxide turns lime water milky when passed through it. This is the characteristic test for carbon dioxide gas.
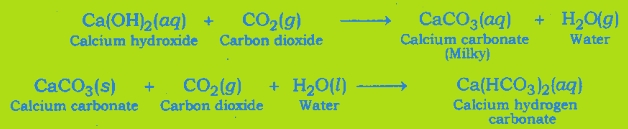
The gas evolved because of reaction of the acid with metal carbonate or metal hydrogen carbonate turns lime water milky. This shows that the gas is carbon dioxide gas.
Acids
- Strong Acids
An acid which is completely ionised in water and produces (H+) is called Strong Acid.
Examples: Hydrochloric acid (HCl), Sulphuric acid (H2SO4), Nitric acid (HNO3)
- Weak Acids
An acid which is partially ionised in water and thus produces a small amount of hydrogen ions (H+) is called a Weak Acid.
Example: Acetic acid (CH3COOH), Carbonic acid (H2CO3) , When a concentrated solution of acid is diluted by mixing water, then the concentration of Hydrogen ions (H+) or hydronium ion (H3O–) per unit volume decreases.
Bases: Bases are bitter in taste, have soapy touch, turn red litmus blue and give hydroxide ions (OH–) in aqueous solution.
Examples: Sodium hydroxide (caustic soda) – NaOH
Calcium hydroxide – Ca(OH)2
Potassium hydroxide (caustic potash) – (KOH)
Properties of Bases:
1.Have a bitter taste.
2.Turns red litmus blue.
3.Conducts electricity in solution.
4.Release OH– ions in Aqueous Solution
Types of bases: Bases can be divided in two types – Water soluble and Water-insoluble.
The hydroxide of alkali and alkaline earth metals are soluble in water. These are also known as alkali.
For example; sodium hydroxide, magnesium hydroxide, calcium hydroxide, etc.
Chemical properties of bases:
(i) Reaction of Base with Metals: When alkali (base) reacts with metal, it produces salt and hydrogen gas.
Alkali + Metal → Salt + Hydrogen
(ii) Reaction of Base with Oxides of Non-metals: Non-metal oxides are acidic in nature. For example; carbon dioxide is a non-metal oxide. When carbon dioxide is dissolved in water it produces carbonic acid.
Therefore, when a base reacts with non-metal oxide, both neutralize each other resulting respective salt and water.
![]()
Base + Non-metal oxide → Salt + Water
(iii) Neutralisation Reaction: An acid neutralizes a base when they react with each other and respective salt and water are formed.
![]()
Acid + Base → Salt + Water
(iv) Reaction of Acid with Metal Oxides: Metal oxides are basic in nature. Thus, when an acid reacts with a metal oxide both neutralize each other. In this reaction, the respective salt and water are formed.
![]()
Acid + Metal Oxide → Salt + Water
Common in all bases: A base dissociates hydroxide ion in water, which is responsible for the basic behaviour of a compound.
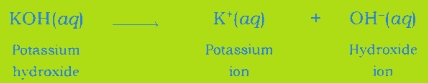
Example: When sodium hydroxide is dissolved in water, it dissociates hydroxide ion and sodium ion.
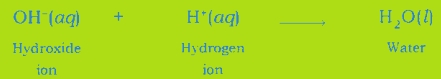
Neutralisation Reaction: When an acid reacts with a base, the hydrogen ion of acid combines with the hydroxide ion of base and forms water
Dilution of Acid and Base: The hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows the concentration of acid or base.By mixing of acid to water, the concentration of hydrogen ion per unit volume decreases. Similarly, by addition of base to water, the concentration of hydroxide ion per unit volume decreases. This process of addition of acid or base to water is called Dilution and the acid or base is called Diluted.The dilution of acid or base is exothermic. Thus, acid or base is always added to water and water is never added to acid or base. If water is added to a concentrated acid or base, a lot of heat is generated, which may cause splashing out of acid or base and may cause severe damage as concentrated acid and base are highly corrosive
Examples
Acids
1.Hydrochloric acid (HCl)
2.Sulphuric acid (H2SO4)
3.Nitric acid (HNO3)
Bases
1.Sodium hydroxide (NaOH)
2.Potassium hydroxide (KOH)
3.Calcium hydroxide (Ca(OH)2)
Bronsted Lowry theory
1.A Bronsted acid is an H+ (aq) ion donor.
2.A Bronsted base is an H+ (aq) ion acceptor.
Example
In the reaction: HCl (aq) + NH3 (aq) → NH+4(aq) + Cl− (aq)
HCl – Bronsted acid and Cl− : its conjugate acid
NH3 – Bronsted base and NH+4 : its conjugate acid
Physical test
Given are two possible physical tests to identify an acid or a base.
a. Taste
An acid tastes sour whereas a base tastes bitter.
The method of taste is not advised as an acid or a base could be contaminated or corrosive.
b. Effect on indicators by acids and bases
An indicator is a chemical substance which shows a change in its physical properties, mainly colour or odour when brought in contact with an acid or a base.
Below mentioned are commonly used indicators and the different colours they exhibit:
a) Litmus
In a neutral solution – purple
In acidic solution – red
In basic solution – blue
b) Methyl orange
In a neutral solution – orange
In acidic solution – red
In basic solution – yellow
c) Phenolphthalein
In a neutral solution – colorless
In acidic solution – remains colorless
In basic solution – pink
Acid-Base Reactions & Reactions of acids and bases
a) Reaction of acids and bases with metals
Acid + active metal → salt + hydrogen + heat
2HCl + Mg → MgCl2 + H2 (↑)
2NaOH + Zn → Na2ZnO2 + H2 (↑)
b) Reaction of acids with metal carbonates and bicarbonates
Acid + metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl + CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
c) Neutralisation reaction
1. Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2. Reaction of non-metal oxides with bases
Non-metal oxides are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH + CO2→ Na2CO3 + H2O
Base:
Bases undergo neutralisation reaction with acids.
They are comprised of metal oxides, metal hydroxides, metal carbonates and metal bicarbonates.
Most of them are insoluble in water.

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
