- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Dobereiner’s Triads
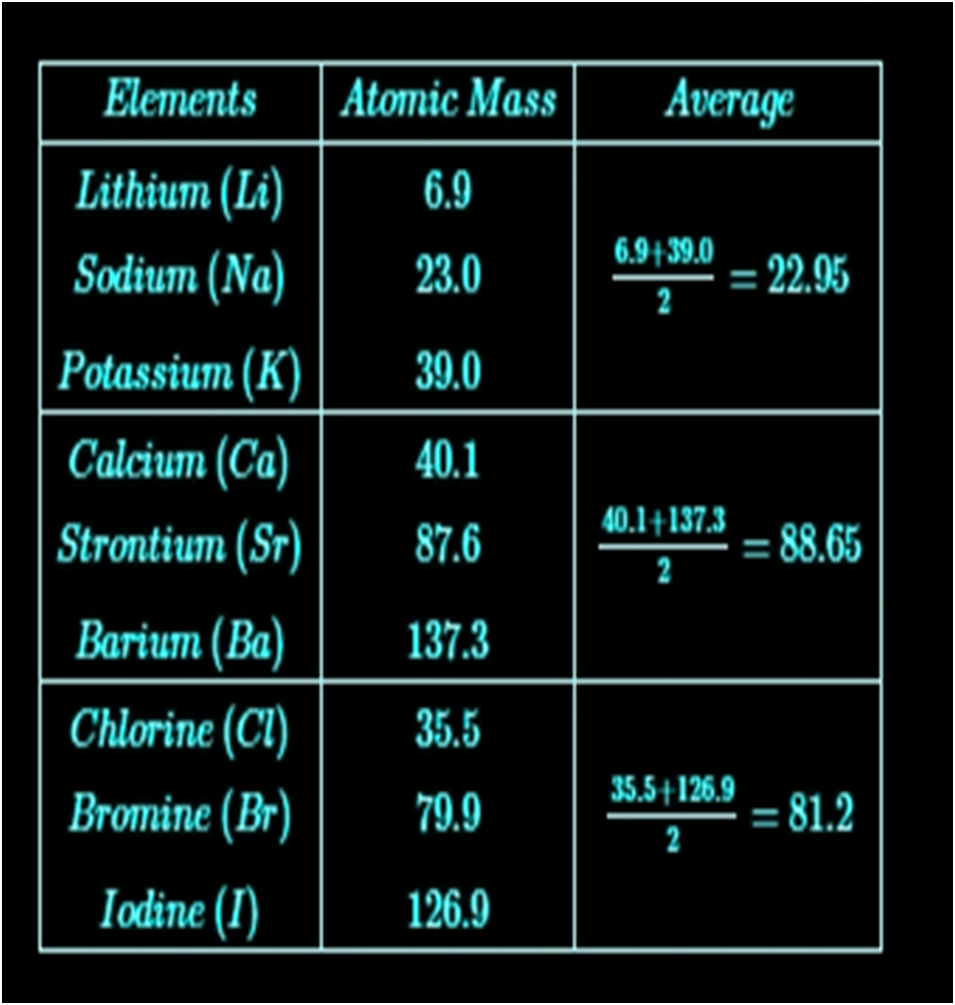
Dobereiner arranged a group of three elements with similar properties in the order of increasing atomic masses and called it a triad. He showed that the atomic mass of the middle element is approximately the arithmetic mean of the other two. But, Dobereiner could identify only the following three triads from the elements known at that time.
Newlands’ Law of Octaves
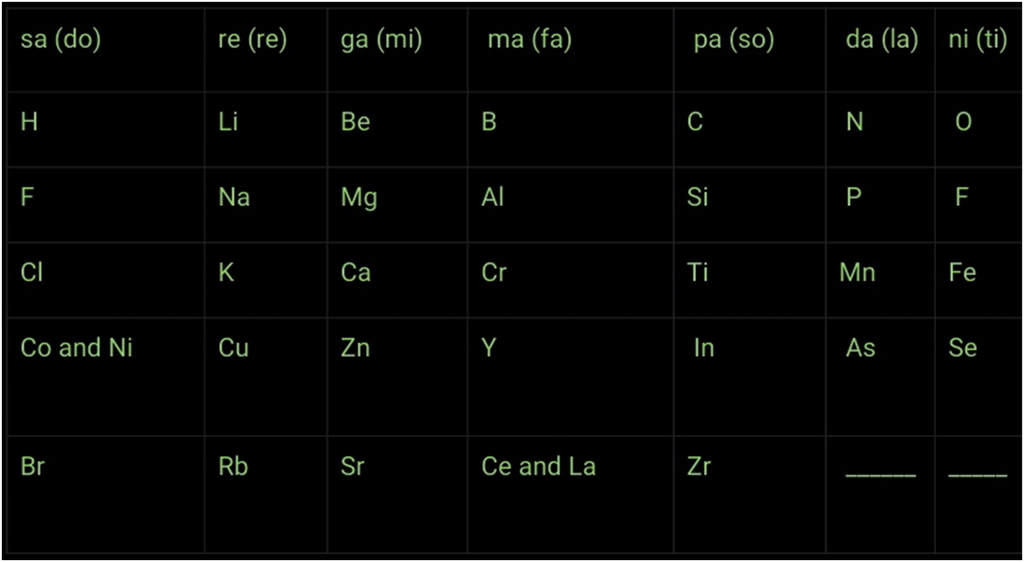
Assumptions and Limitations:
1. The law was applicable for elements with atomic masses up to 40.
2. Properties of new elements discovered did not fit into the law of octaves.
3. In a few cases, Newlands placed two elements in the same slot to fit elements in the table.
4. He also grouped unlike elements under the same slot.

 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
 Grow Career Publication
Grow Career Publication
